Sign in |
Register |
 Cart |
Help center |
中文版
Cart |
Help center |
中文版
 022-58105045
022-58105045
新闻中心
NEWS CENTER
SUNGENE biotech gains Medical Device Registration Certificate
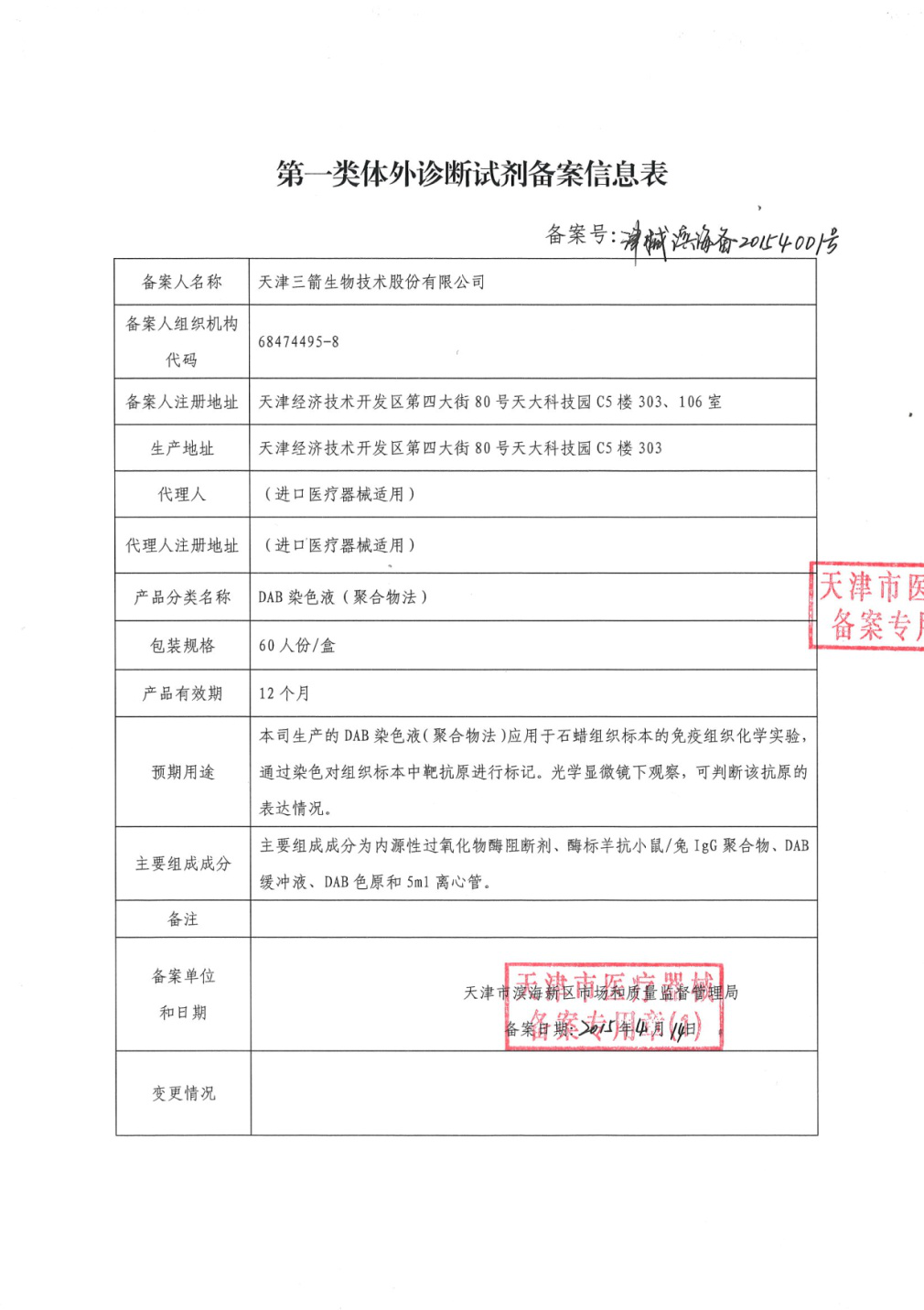
Warmly congratulate Tianjin Sungene biotech on obtaining Medical Device Registration Certificate issued from food and drug administration of Tianjin binhai new district. After examination, "DAB staining solution (polymer method)" produced by company is permitted to register in line with market accessing provisions of medical device, it is valid until May 29th, 2018. The product can react with primary antibody of mouse or rabbit, it is applicable as "clinical diagnosis" product; It can also be used in immunohistochemistry, it is easy to operate, highly sensitive and no cross-reactivity of endogenous biotin, etc..
In a long-term, the company is committed to studying, developing, producing and selling of biology reagents, it's a high-tech enterprise in the bio-pharmaceutical industry with capability of independent innovation. The acquisition of "DAB staining solution (polymer method)" Medical Device Registration Certificate, demonstrates company's strong R & D ability, expand the products types in company, further enhance company's market competitiveness.
Previous chapter: Sungene biotech gain...
Next chapter: SUNGENE expands the ...Publication date: 2015-12-18